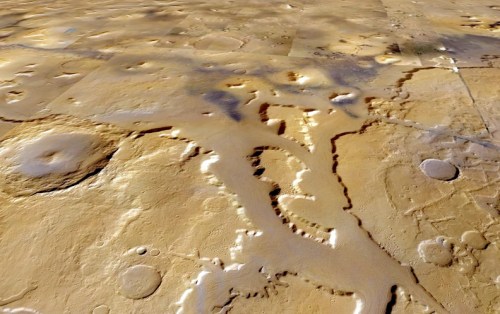
My last post had me still in San Francisco at the NSTA national conference. That was March. Now it’s May, and I don’t quite know what happened to April. Let me try to catch up on myself and this project.
Back in San Francisco, I had just been awarded 3rd Place in the Mars Education Challenge by Bill Nye (yeah, that guy) and by the Explore Mars Foundation. That was on Thursday, March 10. On Friday, March 11 I attended a number of excellent presentations including one on an online student science project from Mt. Pisgah Observatory to classify stars based on their absorption spectra. Thousands of photographic plates with the stars’ light refracted into spectra have been digitized and made searchable. A spectrum from a star can be compared against standard spectra for major stellar classes and subclasses. I will incorporate this activity into my astronomy classes.
My second session was to be over in the Moscone Center on how to use the iPad in science education, a subject I’ve talked about here before, but when I got there the room was packed and people were standing in the aisles and flowing into the hall. This isn’t too surprising – as I saw later that day at the nearby Apple Store, the lines were very long (all the way around the block) and Apple employees were handing out fruit (apples, of course, and oranges) and granola bars just so people wouldn’t pass out from lack of food for waiting so long. The reason: the iPad 2 came out that day.
Instead of the iPad session, I went next door to a good session on project-based learning in the classroom, where a junior high in Lincoln Parish in Louisiana has created a program that is completely project based, yet covers all core curriculum. I found out more about it from the presenters afterward.
I had planned on going to more sessions, but since I was in the Moscone Center it seemed a good time to check out the dealers exhibit. The exhibit hall is a huge, cavernous space with the big name companies jockeying for prime spaces by the main entrance and smaller companies along the aisles in the back corners. I was ostensibly looking for the Explore Mars booth, but I systematically covered the floor and visited anything that caught my eye, picking up a lot more materials to take home than I really wanted to. I was glad I left some space in my suitcase. I finally found the Explore Mars booth on the NSTA aisle (the competition was sponsored by NSTA) and I reported in to Artemis and Chris, who said that the first place winner had arrived and that we would have another small presentation later that afternoon.
I went to lunch, finding a place about a block away called Mel’s Diner. As I sat down at a stool at the counter, the person sitting next to me turned to me and said, “Well, Dave, how are you?” It was Eric Brunsell, who now teaches at the University of Wisconsin at Oshkosh. I first got to know Eric through the NASA/JPL Solar System Educators Program (SSEP), the same group I had dinner with the night before. Eric was with Space Explorers, the group that managed the training sessions for SSEP. We had a good talk about what he’s been doing and on the problems currently being faced by teachers in Wisconsin, where the governor is trying to destroy the teachers union and cut teacher benefits and retirement.
Back at the Moscone Center, I reported in at the booth and met Howard Lineberger, the first place winner. Andrew Hilt (2nd place) and Howard and I stood with Artemis and Chris and officials from NSTA for more photo ops, and were interviewed by Chris on camera on our feelings about Mars exploration. Chris and Artemis had to go to another reception, so they asked us to man the booth until the end of the day. Andrew and I talked to anyone who was interested about the competition and showed them our lesson plans.
Afterward, we decided to walk up to Chinatown for supper. We headed to my hotel to drop off my stuff, then to Andrew’s hotel, then we walked up Nob Hill. We wound up going too high (it is quite a steep hill and we got a good leg stretching) and had to wander back down to the east into Chinatown. I found a really good Chinese bakery, where we sampled the yedz (coconut rolls) and I later bought a koushu binggan (kind of a graham cracker cookie). We found a promising SzeChwan restaurant and had supper. I found out the Andrew and Eric Brunsell are friends and have worked on common projects together. Small world! We also compared notes on our astronomy classes. We walked back down to where our hotels were, and I said goodbye (Andrew is heading home tomorrow). I found a good souvenir cable car ornament for my wife, then headed back to my hotel.