For the last week, I’ve been busy preparing for my classes at Walden School, including inventorying the science lab room (which is also my classroom) and planning out my course schedules. I’ll be teaching two sections of Chemistry, one of Astronomy, one of Computer Technology (a basic computer literacy course required in Utah), a section of Media Design, and a section of Video Production. This is, for me, a perfect schedule. In the meantime I’ve also been preparing a series of maps and 3D images of the Tintic Mining District, focusing on the ore deposits and the various mines located there. I’ve also prepared the script for this section of the video, which I have pasted below:
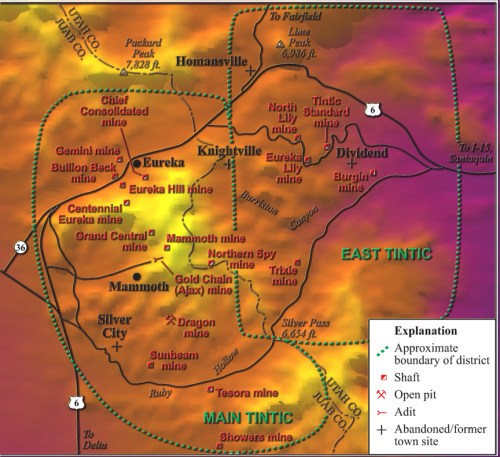
MInes and Roads in the East Tintic Mtns.
Tintic Geology
To understand how the ore bodies in the Tintic District were deposited, we have to start about 800 million years ago in the Precambrian Period when the western portion of the North American craton rifted away from the rest of the continent along a line where the Wasatch Front now lies – this Wasatch Line has been an important hinge line in Utah’s geology ever since. For the next 600 million years, a sequence of ocean sediments including dolomite, limestone, shale, and sandstone were deposited off the coast in the geosyncline that would become western Utah. Beginning 150 million years ago, Nevada and then western Utah were uplifted as the Farallon tectonic plate was pushed under North America. Like a throw rug being wrinkled up as it’s pushed over a hardwood floor, western Utah was folded by thrust faults into a large mountain range during the Sevier orogeny about 70 million years ago. This thrusting continued across eastern Utah and into Colorado and Wyoming during the Laramide orogeny, building up the Uintah and Rocky Mountains.
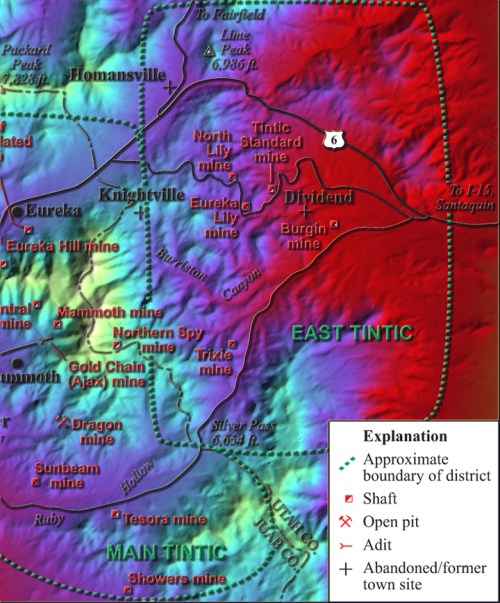
Mines in the eastern portion of the Tintic Mining District
Then, about 50 million years ago, the Farallon plate began to collapse from underneath the continent. As it peeled away, a wave of volcanism moved from east to west across Colorado and Utah. Intrusive laccoliths rose to the surface, bulging up the LaSal and Henry Mountains in eastern Utah and forming explosive calderas in several places in western Utah. About 35 million years ago, a series of calderas formed in the area that would become the Tintic Mountains. A large andesitic volcano rose up from eruptions of ash and tuft.

Ore samples from the Tintic Standard Mine, eastern district.
About 31.5 million years ago, the volcano collapsed as the intrusive magma began to cool. Mineral rich fluids were injected into the surrounding limestone, quartzite, and dolomite as replacement beds. The hot magma caused the carbonate rocks to decompose; for example, limestone turns into lime or calcium oxide and carbon dioxide gas when heated. This left large cavities that then filled up with the mineral-laden magmas. These deposits are called stopes, such as the famous Oklahoma stope of the Chief Consolidated mine. The carbon dioxide released from the decomposing limestone and dolomite in turn dissolved into the hot magma, making it a kind of lava champagne, and reacting with it to form various exotic minerals, some of which are found nowhere else.

More ore samples from the Tintic District
The primary ore-bearing minerals in the Tintic District are enargite, tetrahedrite, galena, sphalerite, pyrite, marcasite, and native gold, silver, and copper. But many more minerals are present, including unusual minerals that blend copper, silver, tellurium, arsenic, sulfur, carbonates, hydrodixes, etc. At the Centennial Eureka mine, over 85 different minerals have been identified, ranging from common pyrite, malachite, and azurite to minerals found only here. It is the type locality (where the mineral was first identified) for leisingite, frankhawthorneite, jensenite, juabite, utahite, and eurekadumpite. Other rare minerals include xocomecatlite, carmenite, adamite, duftite, and mcalpineite.
These mineral deposits occurred around the edges of the caldera and formed the five large ore zones of the main Tintic District. The Gemini Ore Zone runs to the west of Eureka south to the north edge of Mammoth Gulch. The Gemini, the Bullion Beck and Champion, the Eureka Hill, and the Centennial Eureka mines (known collectively as the Big Four) are located on this zone.
The Chief-Mammoth Ore Zone begins under the center of Eureka and extends due south across the mountain to the east end of Mammoth Gulch. The Chief Consolidated mine is located on the richest ore body, which is right under the center of Eureka city; up the hill is the Eagle and Blue Bell mine, named for the beautiful deposits of azurite found inside. Further south over the top of Eureka Peak lie the Grand Central, Mammoth, Apex, and Gold Chain mines that are also part of this deposit.
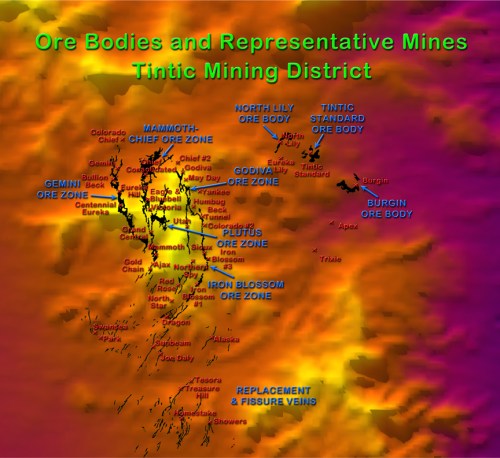
Ore Zones and Major Mines of the Tintic Mining District
The Plutus Zone branches off of the Chief-Mammoth Zone high up in the Tintic Mountains. The Godiva Zone starts just east of Eureka and runs southeast in a curve where it joins the Iron Blossom Zone, which continues in a curve south and then southwest. Some mines in these zones include the Godiva, May Day, Humbug, Beck Tunnel, Sioux, and Iron Blossom mines.
In the eastern section of the Tintic District, several zones of minerals were deposited and were among the last to be discovered because they are overlain by 400 feet of igneous rock. These bodies include the Burgin ore body, the Tintic Standard, and the North Lily bodies. Other bodies are located at the Apex and Trixie mines.
In the southern section of the Tintic District, the large replacement bodies give way to smaller fissure veins that are only two feet wide on average but can be up to 4000 feet long. Here, the mineral-bearing magma was injected into cracks and fault lines already existing in the host rocks. The Dragon mine is the only true open pit mine in the area; it sits on top of a network of fissure veins at the south end of the Iron Blossom Zone. Other mines in the area include the Swansea and Sunbeam mines at Silver City, the Tesora and Treasure Hill mines at Ruby Gulch, and the Showers mine at Diamond Gulch.

More ore samples from the Tintic Standard Mine
The final chapter in the area’s geomorphology began about 17 million years ago when normal faulting created the Basin and Range province, lifting up blocks to form the mountain ranges of Utah and Nevada, including the East Tintic Mountains. Other blocks sank to form the valleys, such as the Tintic Valley. Erosion has exposed the ore bodies in many places, including the outcropping that George Rust stumbled over in 1869. It was to become the Sunbeam Mine.
Read Full Post »